 Silicone (or siloxane) polyether copolymers
Silicone (or siloxane) polyether copolymers are commonly composed of a silicone polymer component chemically combined with one or more organic polyether components. Polar organic groups such as polyoxyalkylene, quaternary ammonium salts, and betaines have been chemically bound to the polysiloxane backbone to form siloxane
surfactants. The chemical combination of a polysiloxane and a polyether may occur either with the formation of hydrolytically stable silicon-carbon bonds (Si-C) or with hydrolytically unstable silicon-oxygen-carbon bonds (Si-O-C). Which of these types of linkages is desired in the silicone polyether copolymer determines the choices of reagents necessary for the chemical coupling reaction as well as the physical properties of the resulting copolymer. Most of the products made by Hangzhou
Top Win Technology Development Co., Ltd. are Si-C linked Copolymers of SILICONE POLYETHER COPOLYMERS.
The formation of a Si-C bond is accomplished by hydrosilylation of a silicon hydride functional polysiloxane with an allyloxy polyether. Rake or pendant type silicone polyethers are the most common structures and are represented by the formula Me3SiO(Me2SiO)x(RMeSiO)ySiMe3, where Me represents a methyl group and R represents the polyether functionality. The levels of x and y and ratio x/y can vary widely and determine the physical, chemical, and surface properties of the resulting copolymer. The form of R can vary significantly as well. It can be represented by an (EO)m(PO)n(BO)o notation, where EO represents an ethylene oxide (CH2CH2O) unit, PO represents a propylene oxide (CH2CHCH3O) unit, and BO represents a butylene oxide (CH2CH(CH2CH3)O) unit.
A wide variety of structures are possible, ranging from high molecular weight siloxane polymers where x is large, to those containing no dimethylsiloxane units.
The simplest cases of these types of structures are the following discrete compounds ( Figures 1,2,3,4).
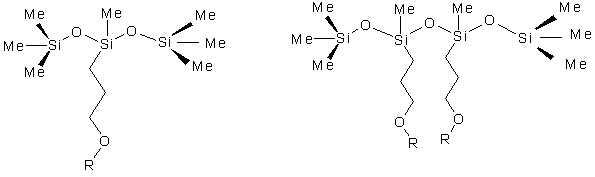
Figure (1) Figure (2)
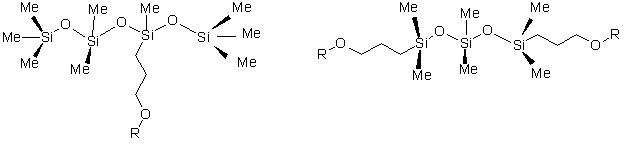
Figure (3) Figure (4)
Where Me represents a methyl group and R represents a polyether substitution. Compound 1 is a trisiloxane based surfactant, Compound 2 has multiple polyether rake or pendant substitutions, Compound 3 has both dimethylsiloxane and monomethylsiloxane/polyether rake substitutions, and Compound 4 is an ABA type block copolymer. Compounds 2, 3, and 4 are the simplest cases of a particular class of materials. More complex analogs can be found with multiple repeat units of dimethylsiloxane or monomethylsiloxane/polyether. Branched polymers can be obtained by the incorporation of siloxane T units into the structure. The simplest case of such a T structure is shown in Figure (5), where X represents the continuation of the polysiloxane chain.
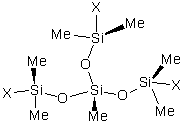
Figure (5)
Top Win is one of the leading & professional manufacturers with more than 20 years' experience, have domestic top-level engineers in silicone surfactant industry. We specialized in producing all kinds of SILICONE BASED new materials and specialized in research, production, sales and marketing in silicone-based performance materials. Provide good OEM&ODM service.Our products are mainly used in polyurethane foam, Agriculture,Coatings and Inks, Leather & Textiles, Pulp and Paper, Cosmetic industry, etc.



